
Manufacturers must produce a variety of ATMPs quickly and efficiently. Enter the multi-product ATMP facility.
The rapidly growing advanced therapy medicinal product (ATMP) market gives hope to people suffering from previously untreatable and rare diseases. But, to make that hope a reality, manufacturers must find a way to produce ATMPs quickly, efficiently and at scale.
Enter the multi-product ATMP facility.
The state of the ATMP industry
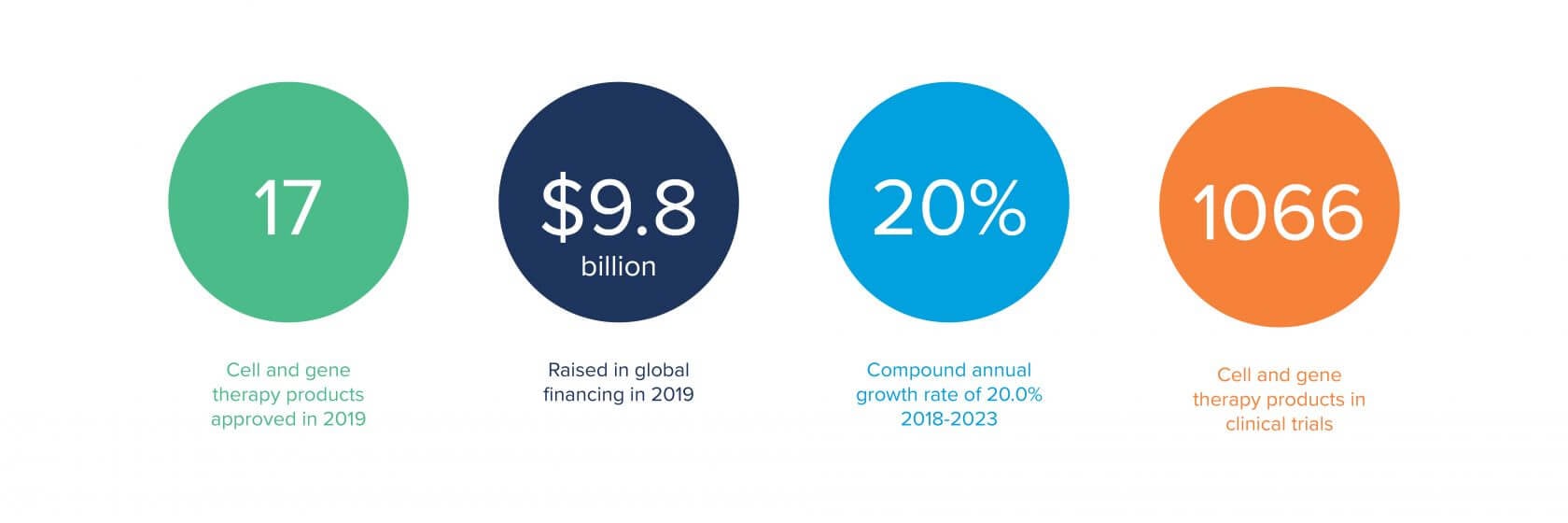
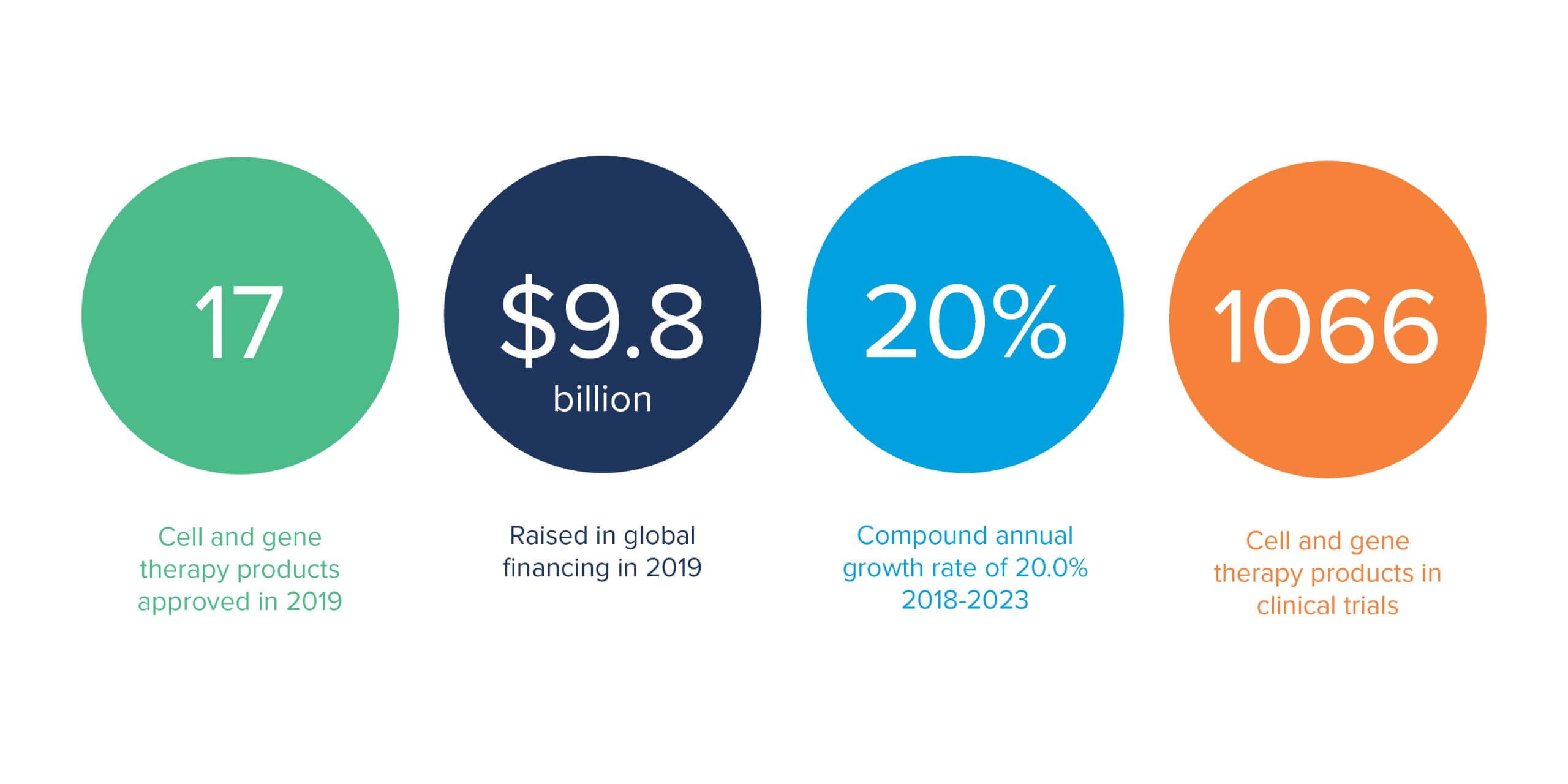
Why is multi-modal important at this juncture in the industry? We’re seeing more and more mergers and acquisitions of smaller start-up companies by much larger, established biotech and pharmaceutical companies. Where they typically had a limited number of modalities, these companies are now dealing with potentially six or more modalities of product lines.
And, at the same time, there’s some uncertainty in the marketplace about which modalities are going to dominate. Is there going to be a shift or trend toward one type of modality versus another?
It’s a dynamic environment where companies are trying to build manufacturing facilities, but they’re not sure about what the future of their pipeline is going to look like.
A logical way to adapt to an evolving pipeline and “future proof” your investment is a multi-product facility.
Why multi-product ATMP facilities work
The right approach to a multi-modal facility doesn’t mean combining all products under one roof. It doesn’t lump together products with wildly different platforms, scales and equipment technologies.
Multi-modal manufacturing optimizes product combinations based on manufacturing and regulatory similarity and compatibility.
In this webinar, originally shared through Cell and Gene Therapy Insights, we explain the three product types that provide the best possibility for an effective and efficient multi-product facility. We discuss the science and manufacturing considerations that make these products compatible. And we explore the design that will make this flexible facility a reality.
Achieve appropriate scale, faster speed-to-market, lower costs and manufacturing flexibility with a multi-product ATMP facility. Listen to our webinar to find out how.


