Vascudyne Announces Presentation of First Clinical Results of TRUE AVC™ in Hemodialysis Access at the VASA Conference

Vascudyne, Inc., a biotechnology trailblazer in regenerative medicine, announced today that the first clinical results of TRUE AVC™ acellular vascular conduit in hemodialysis access will be presented at the Vascular Access Society of the Americas (VASA) conference in Charleston, South Carolina at 11:20 am EDT on June 10, 2022.
The presentation, titled “First in Human Evaluation of a Novel Biologic Regenerative Vascular Conduit for Hemodialysis Access”, will be made by Vascudyne’s medical advisor Monnie Wasse, MD, MPH, FASN, Professor of Medicine in the Division of Nephrology and Department of Internal Medicine at Rush University Medical Center. Dr. Wasse serves as Vice-Chairperson for Clinical Operations for the Department of Medicine and is also the Director of Interventional Nephrology, focused on the planning and maintenance of dialysis vascular access for patients with kidney diseases. Dr. Wasse will present 6-month results and preliminary evidence of the safety and effectiveness of the device as a conduit for hemodialysis vascular access.
Vascudyne’s TRUE™ Tissue products are unique, 100% biological, and become the patient’s own living and functional tissue. Nothing synthetic or artificial is ever used in the manufacturing process, in contrast to other regenerative medicine cardiovascular devices with synthetic polymer-based scaffolds that slowly degrade in the body and may lead to adverse immune response.
“Dr. Wasse brings extensive experience and clinical expertise to our medical advisory board, and we are excited she is presenting the promising initial results from the TRUE AVC studies,” said Mark Stenoien, Vascudyne’s Chief Regulatory and Clinical Science Officer.
Vascudyne announced in February its relocation to a high capacity GMP manufacturing facility to support TRUE AVC manufacturing through commercialization. “We are excited to share our first clinical results with our TRUE Tissue technology in hemodialysis access and have been working on developing our platform technology to address additional cardiovascular diseases” said Dr. Zeeshan Syedain, Vascudyne’s Chief Scientific Officer. “Our move to the new facility dramatically increased our GMP manufacturing space and added a dedicated cleanroom that is poised to support our ongoing TRUE AVC clinical studies all the way to commercialization,” added Dr. Syedain.
TRUE AVC is not available for commercial sale.

Dr. Monnie Wasse, MD, MPH, FASN
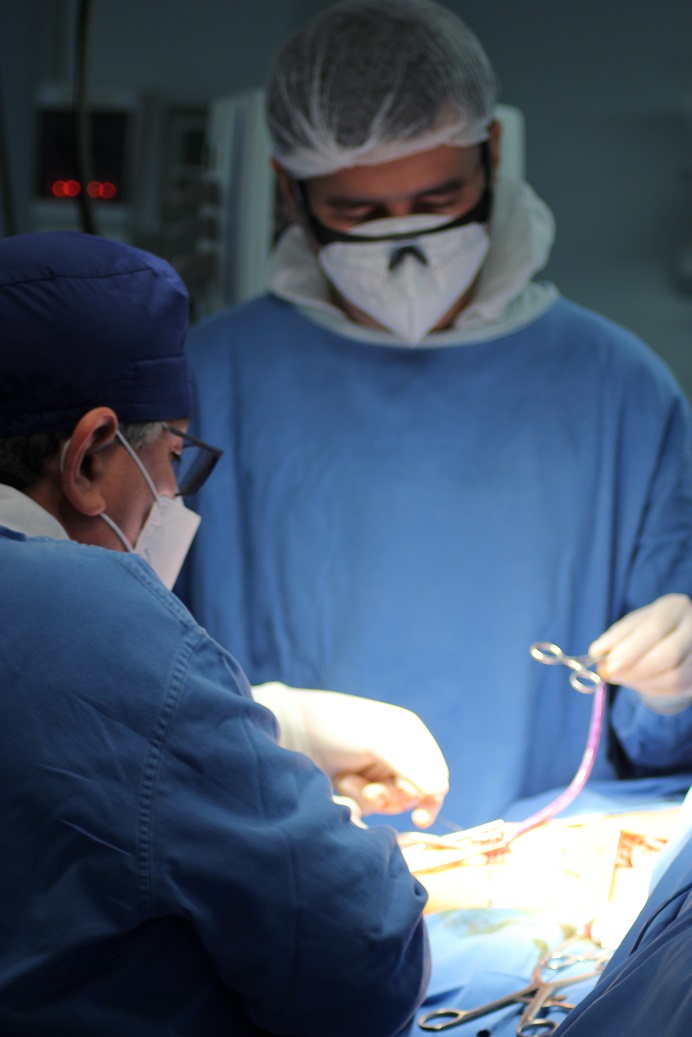
Vascudyne’s Clinical Collaborators at Sanatorio Italiano in Asuncion, Paraguay

Vascudyne’s TRUE AVCTM for Hemodialysis Access
About Vascudyne
Headquartered in the heart of Medical Alley in Minnesota, Vascudyne is on a mission to improve patient care with regenerative biomaterials that are inspired by nature. Vascudyne, a privately held company founded in 2014, uses the TRUE™ Tissue technology to develop TRUE to Nature™ biomaterials for soft tissue repair and replacement. For more information, please visit https://www.vascudyne.com/.
About TRUE Tissue Technology
TRUE™ Tissue is developed from cells isolated from donor tissue and is 100% biological. There are no synthetic materials or chemical fixation used, and implanted tissues are completely cell-derived and acellular. The TRUE Tissue technology can be readily shaped into tubes, sheets, and other geometries making it suitable for many soft tissue applications, is mechanically comparable to native tissues, and is a ready to use, off-the-shelf allograft.
Forward Looking Statements
This announcement contains forward-looking statements. Such statements may include, without limitation, statements identified by words such as "projects," "may," "will," "could," "would," "should," "believes," "expects," "anticipates," "estimates," "intends," "plans," "potential" or similar expressions. These statements relate to future events or Vascudyne’s clinical development programs, reflect management’s current beliefs and expectations and involve known and unknown risks, uncertainties and other factors that may cause Vascudyne’s actual results, performance or achievements to be materially different. Vascudyne undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future presentations or otherwise, except as required by applicable law.
Contact Details
Sandy Williams, Marketing Director




